The medical industry has long required packaging solutions that meet stringent hygiene, safety, and functional standards. With the increasing demand for precision, scalability, and sterility in medical product packaging, traditional manufacturing methods have struggled to keep pace. The introduction of the Medical Packaging Mould has significantly transformed this aspect of production. Designed specifically for molding medical-grade packaging materials, the Medical Packaging Mould addresses several longstanding manufacturing challenges related to consistency, efficiency, cleanliness, and regulatory compliance.
Enhancing Sterilization Compatibility
Medical products are often sterilized using methods such as gamma irradiation, ethylene oxide, or steam autoclaving. Packaging materials and designs must be compatible with these processes. Prior to the use of specialized Medical Packaging Moulds, manufacturers faced difficulties in achieving packaging shapes and thicknesses that could endure sterilization without deforming or losing barrier properties. The Medical Packaging Mould enables the production of containers and seals that maintain integrity through multiple sterilization methods, ensuring product safety and regulatory compliance.
Addressing Cleanroom Manufacturing Requirements
Medical packaging must be produced in environments that prevent contamination. Standard molds and tooling were often not suited for cleanroom use due to issues like particulate generation, poor material compatibility, or lubricant leakage. The Medical Packaging Mould is engineered with cleanroom compatibility in mind. Features such as self-lubricating components, minimal moving parts, and corrosion-resistant materials help maintain cleanliness standards. The use of Medical Packaging Moulds has thus enabled more widespread adoption of Class 7 and Class 8 cleanroom production lines in the packaging sector.
Supporting Scalability in Production
As global demand for medical devices and pharmaceuticals continues to rise, manufacturers must scale production without sacrificing quality. Traditional tooling systems were often unable to meet high output demands without frequent maintenance or quality degradation. The Medical Packaging Mould, by contrast, is built for high-cycle operation and long production runs. Its robust construction materials, optimized cooling channels, and balanced cavity layout allow manufacturers to produce millions of identical units with minimal downtime and consistent quality.
Streamlining Validation and Compliance
Medical packaging must adhere to strict regulatory requirements such as those outlined by the U.S. FDA, the EU's MDR, and ISO 11607 standards for packaging of terminally sterilized medical devices. The Medical Packaging Mould is designed with these standards in mind, helping manufacturers streamline the validation process. Because these molds offer high reproducibility and are typically supported by documented design and material traceability, they simplify the regulatory validation required for packaging systems.
Improving Material Utilization
Material waste has always been a concern in packaging operations, both from a cost and environmental perspective. Earlier packaging methods often resulted in high scrap rates due to uneven material distribution, poor molding accuracy, or suboptimal design. The Medical Packaging Mould enables more efficient use of medical-grade polymers by ensuring uniform material flow, reduced flash, and optimized part geometry. As a result, manufacturers can lower raw material costs and reduce environmental impact, while maintaining the necessary functional performance.
Enabling Customization and Design Flexibility
Medical packaging often needs to be tailored to specific devices or application contexts. Standard packaging molds were typically inflexible, making it costly and time-consuming to develop new package formats. The Medical Packaging Mould supports modular tooling inserts and digital design integration, making customization more accessible. Whether the need is for a multi-cavity tray, a tamper-evident cap, or a barrier pouch, the mold can be rapidly adapted to meet diverse design specifications.

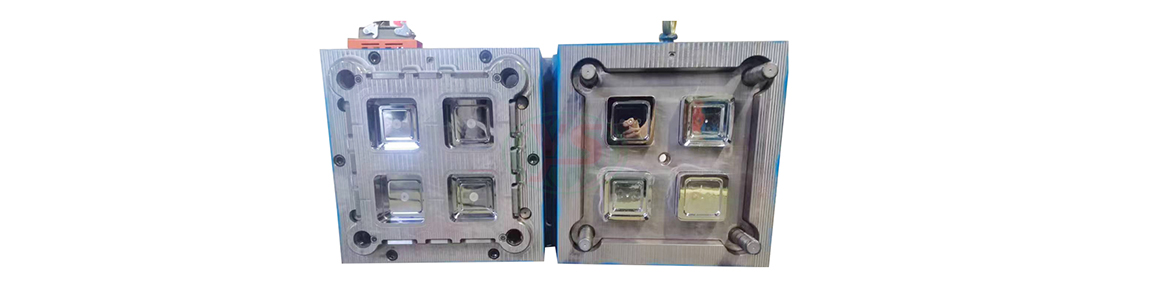
Mold material: S136H 、718H、NAK80、 P20、2738、8407、SKD61、H13Mould cavity number: single cavity, multi cavityProcessing accuracy: 0.05mmMold

We have ISO9001 international quality management system and experience in controlling production costs in China. Therefore, we can help you save 20% -
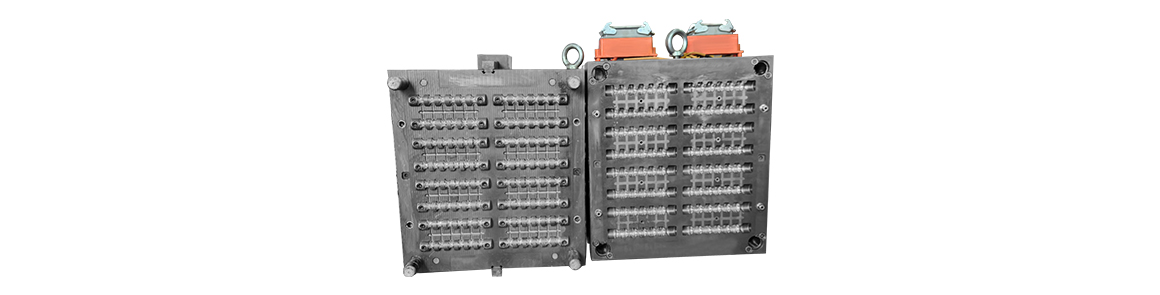
Mold material: S136 HRC48-52Manufacturing process: CNC milling, CNC machining, EDM/wire cuttingWe are equipped with the world's top precision machinin
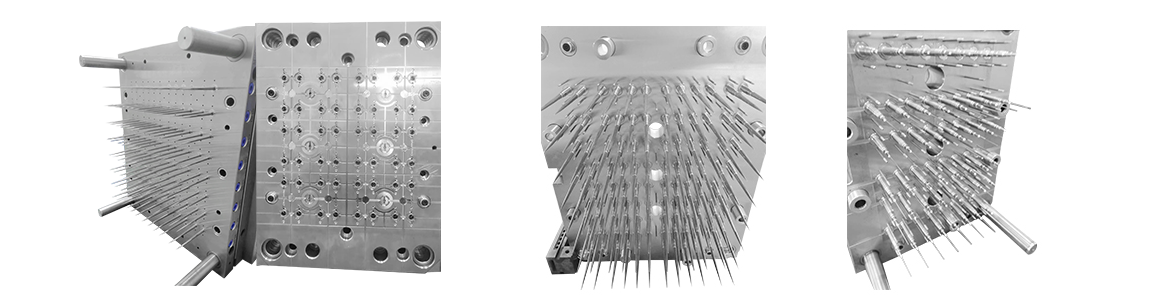
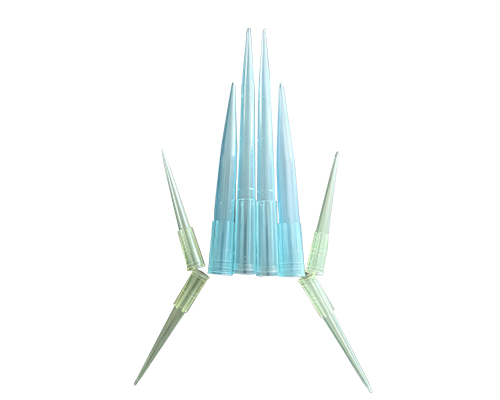
Our liquid transfer gun suction head medicine mould is made of high-quality materials as a whole. There are no burrs at the tip and mouth of the produ

The mold has a short injection cycle, high efficiency, long life, and low cost. Adopting the special flow channel design of the international advanced
This type of mould is assembled from outer cap and an inner anti-theft ring. The outer cap is automatically rotated and pushed out by a hydraulic moto